ACT for ALS is bipartisan legislation that passed in 2021 to enable people living with ALS to access promising investigational therapies when they do not qualify for clinical trial participation. The ACT for ALS was authored by Congress in partnership with I AM ALS, individuals living with and impacted by ALS, medical professionals, scientists, and collaborating organizations after they collectively raised the need for access to clinical research and advocated for an innovative solution for people with a 100% fatal disease with no cure.
Patients are getting access to therapies NOW because of the ACT for ALS!
Why is access to these therapies necessary?
- ALS is 100% fatal for all patients and there are currently very few treatment options that can delay progression.
- The good news is that there are therapies in the research pipeline. However, most people living with ALS are ineligible to participate in a clinical trial as diagnosis is often incredibly delayed due to a variety of issues contributing to missed or misdiagnosis. These delays often cause a person to not qualify for a clinical trial since they are diagnosed outside of the eligibility window.
- The average time for treatments to be tested, approved, and accessible to people living with disease is 10-15 years—people living with ALS will not live that long and do not have time to wait.
- Because of these barriers, the ACT for ALS was written to offer a path for patients to access drugs when early data looks promising, they are not yet approved, and the person with ALS is ineligible to participate in the clinical trial. The law enables up to $75 million annually for government-funded grants that enable biotech companies focused on ALS (usually smaller companies with few resources) to provide access to these treatments in conjunction with phase 3 clinical trials.
- The programs funded through the ACT for ALS are called expanded access programs (EAPs) or expanded access research programs. In addition to enabling immediate access for people who need it most, the law requires participating companies to utilize EAP participant data to support their clinical research.
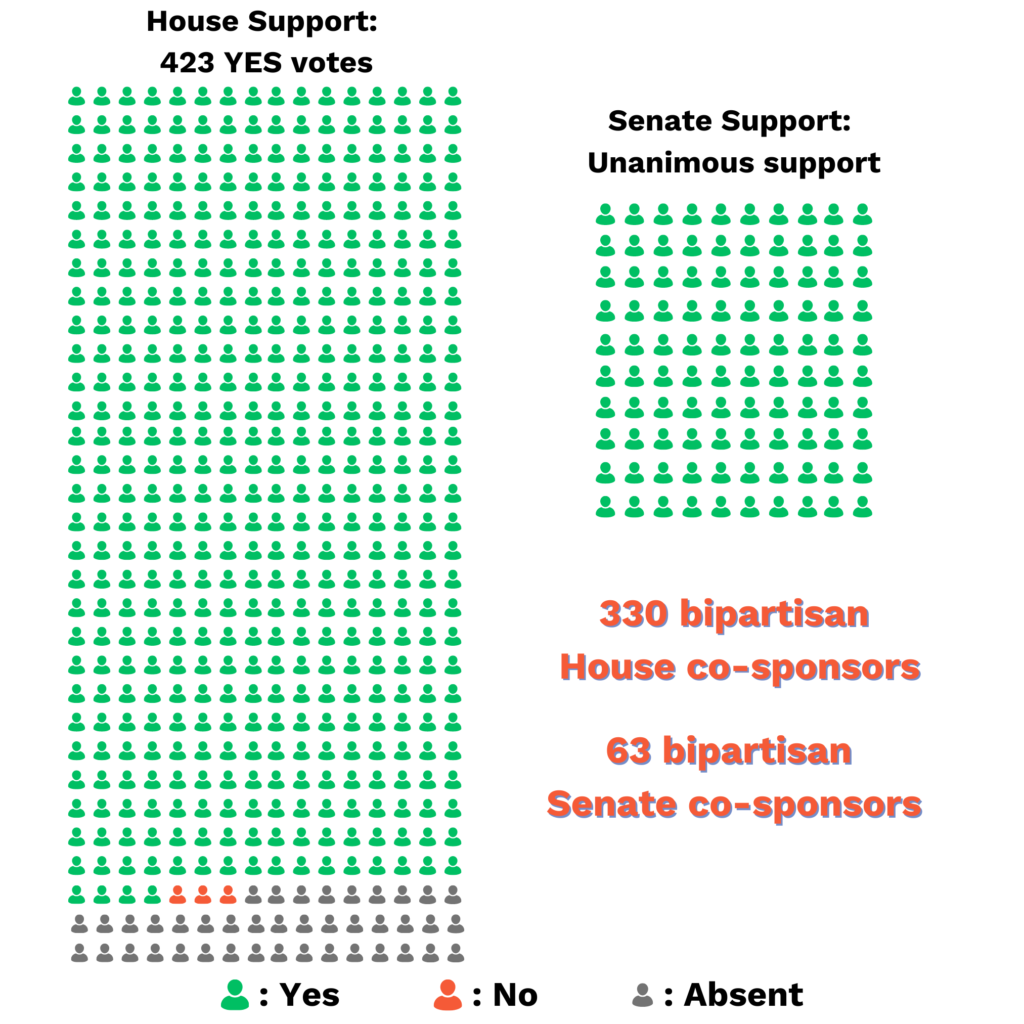
Bipartisan support for ACT for ALS:
- On December 23, 2021, President Biden signed ACT for ALS into law.
- ACT for ALS was the only piece of legislation in 117th Congress to receive a vote with more than 350 signatures across the aisle, and all parties agreed that it must be brought to the floor for a vote.
More ACT for ALS resources:
- YouTube video of President Biden’s remarks as he signs the ACT for ALS into law.
- More from the Food and Drug Administration about ACT for ALS.
- More from the National Institute on Health about ACT for ALS.
- I AM ALS’s press release about the law being passed.
